Binary ionic compounds are made of cations (positively charged) and anions (negatively charged). Cations are formed by metals. Anions are formed by nonmetals. Some cations always have the same charge (fixed charge), and some have variable charges. This unit focuses on only those with fixed charges. Ions of Group 1A always have a charge of 1+. Jq laser 1390. Dietary cation-anion balance and cation source effects on production and acid-base status of heat-stressed cows. Dancing drums strategy. Journal of Dairy Science 75, 2776 – 2786. West, JW, Mullinix, BG and Sandifer, TG 1991.
nombre
Química
Cation And Anion Worksheet
A negatively charged ion, i.e. one that would be attracted to the anode in electrolysis.
‘When two atoms swap electrons to produce a cation and an anion, the two ions are attracted to each other.’- ‘During electrolysis an anion is attracted to the anode (positive electrode).’
- ‘In an ionic compound there are two different types of ions present, the positively charged cations and the negatively charged anions.’
- ‘It is called a cation if a positive charge exists and an anion if a negative charge exists.’
- ‘It's easy to make a high oxidation state in an anion because an anion is electron-rich.’
- ‘All the positively charged ions, or cations, are together on the right side of the collage, and all the negatively charged ions, or anions, are on the left.’
- ‘An ionic compound is composed of a network of ions that results in a three-dimensional matrix of cations and anions.’
- ‘For ions dissolved in water, the rapid exchange of water molecules around the cation usually provides the space for an anion to approach the cation.’
- ‘The cation is positively charged, and the anion is negatively charged.’
- ‘Non-metals accept electrons in forming anions while metals donate electrons to form cations.’
- ‘These water molecules are ripped apart and change into hydroxyl anions, each of which is negatively charged and has one oxygen ion with a proton attached.’
- ‘The molecules or anions attached to the central atom are called coordinating groups or ligands.’
- ‘Cations within the middle solution are attracted to one electrode and anions to the other.’
- ‘A typical reaction of such compounds is to accept an additional electron from an anion or to share electrons with an anion to gain a stable octet.’
- ‘Nitric oxide can react with superoxide anions to produce peroxynitrite anions, thus quenching the biological effects of NO.’
- ‘To balance the influx of positive charge, organic anions, principally malate, and chloride are also accumulated.’
- ‘Crystals of table salt consist of equal numbers of sodium cations and chlorine anions, cation-anion pairs being held together by a force of attraction.’
- ‘Cations and anions can only exist in ionic compounds, nearly all of which are solids at room temperature, or in solution.’
- ‘The functional groups of iron hydroxides may sequestrate some cations and anions.’
- ‘Protons diffuse across a membrane from the anode chamber to the cathode chamber, where they react with the anions to form water or ferrocyanide ions.’
Cation And Anion Worksheet
A negatively charged ion, i.e. one that would be attracted to the anode in electrolysis.
‘When two atoms swap electrons to produce a cation and an anion, the two ions are attracted to each other.’- ‘During electrolysis an anion is attracted to the anode (positive electrode).’
- ‘In an ionic compound there are two different types of ions present, the positively charged cations and the negatively charged anions.’
- ‘It is called a cation if a positive charge exists and an anion if a negative charge exists.’
- ‘It's easy to make a high oxidation state in an anion because an anion is electron-rich.’
- ‘All the positively charged ions, or cations, are together on the right side of the collage, and all the negatively charged ions, or anions, are on the left.’
- ‘An ionic compound is composed of a network of ions that results in a three-dimensional matrix of cations and anions.’
- ‘For ions dissolved in water, the rapid exchange of water molecules around the cation usually provides the space for an anion to approach the cation.’
- ‘The cation is positively charged, and the anion is negatively charged.’
- ‘Non-metals accept electrons in forming anions while metals donate electrons to form cations.’
- ‘These water molecules are ripped apart and change into hydroxyl anions, each of which is negatively charged and has one oxygen ion with a proton attached.’
- ‘The molecules or anions attached to the central atom are called coordinating groups or ligands.’
- ‘Cations within the middle solution are attracted to one electrode and anions to the other.’
- ‘A typical reaction of such compounds is to accept an additional electron from an anion or to share electrons with an anion to gain a stable octet.’
- ‘Nitric oxide can react with superoxide anions to produce peroxynitrite anions, thus quenching the biological effects of NO.’
- ‘To balance the influx of positive charge, organic anions, principally malate, and chloride are also accumulated.’
- ‘Crystals of table salt consist of equal numbers of sodium cations and chlorine anions, cation-anion pairs being held together by a force of attraction.’
- ‘Cations and anions can only exist in ionic compounds, nearly all of which are solids at room temperature, or in solution.’
- ‘The functional groups of iron hydroxides may sequestrate some cations and anions.’
- ‘Protons diffuse across a membrane from the anode chamber to the cathode chamber, where they react with the anions to form water or ferrocyanide ions.’
Origen
Si Cation Or Anion
Se Cation Or Anion In Blood
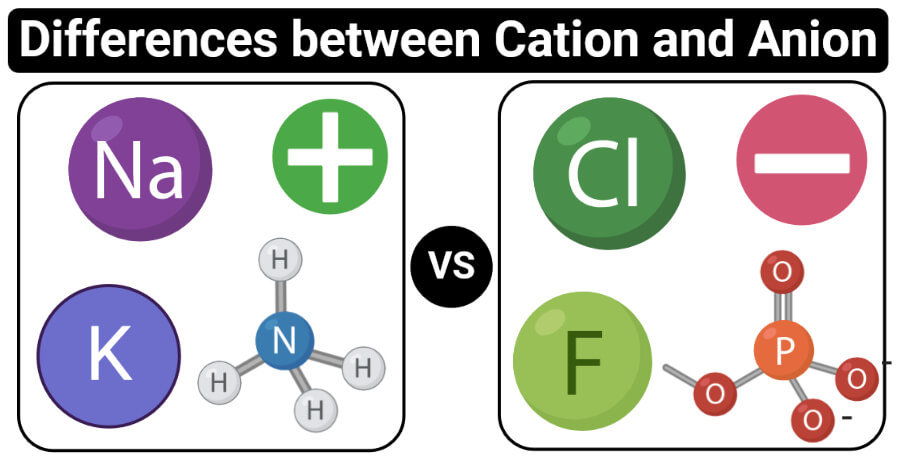
- Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride. A metal reacts with a nonmetal to form an ionic bond. You can often.
- Cation vs anion periodic table It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Halogens always form anions, alkali metals and alkaline earth metals always form cations.

